Abstract
Background: Muscle and fat areas and radiodensities have been linked to clinical outcomes in several malignant and non-malignant conditions. Sarcopenia, defined by low muscle mass, and adipopenia, have been found to be associated with inferior outcomes in several hematologic malignancies, but studies in multiple myeloma (MM) have reported inconsistent results. We designed this study to evaluate the association between muscle and fat areas and radiodensities assessed by 18F-fludeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), and overall survival (OS) in newly diagnosed patients with MM.
Methods: We included 341 patients diagnosed with MM from January 6th, 2010, to June 20th, 2019, who had a PET/CT done at Mayo Clinic within 3 months prior to diagnosis, or after diagnosis, but less 1 month after starting treatment for MM. Body composition analysis was performed on a single-slice CT image at the third lumbar vertebral level that was automatically segmented into skeletal muscle and fat compartments using the BodyCompSlicer software. The following CT attenuation thresholds were used for segmentation: -190 to -30 Hounsfield units (HU) for subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), and intramuscular adipose tissue (IMAT), and -30 to 150 HU for skeletal muscle, which included abdominal wall, paraspinal, and psoas muscles. Manual correction of segmentation was done as necessary by the reviewing radiologist. Muscle and fat cross sectional areas were calculated (cm2) and adjusted for height (m2) to calculate the skeletal muscle index (SMI), SAT index (SAI), VAT index (VAI), IMAT index (IMAI) and total adipose tissue index (TAI). Muscle and adipose tissue radiodensities were calculated as the mean attenuation in HU of the respective compartments.
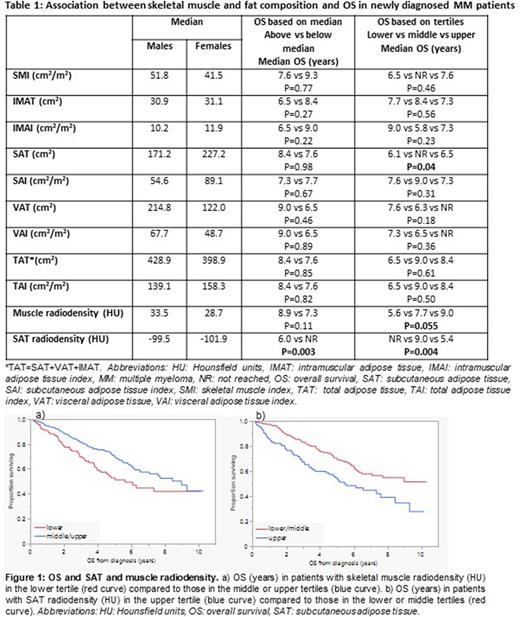
Results: The median age was 65 (interquartile range: 59-72) years; 66% were male. Among all patients, 23%, 65% and 13% had R-ISS stage I, II, and III, respectively. Median follow up was 5.7 (95%CI: 5.4-6.3) years and median OS was 7.7 [95%CI: 6.1-Not reached (NR)] years. The prevalence of sarcopenia ranged from 46% to 56% depending on the SMI sex-specific cutoff used (Prado et al., Martin et al., sex-specific median in our population). There was no association between sarcopenia and disease stage, baseline laboratory, or cytogenetic characteristics. We did not observe an association between IMAT, VAT, or total adipose tissue areas, and OS. Patients in the middle tertile for SAT area (cm2) had better OS compared to those in the lower and upper tertiles (P=0.04). Low muscle radiodensity (lower tertile) was associated with decreased OS; OS was 5.6 vs 9.0 years in patients with muscle radiodensity in the lower vs middle/upper tertiles, respectively (P=0.02) (Figure 1a). High SAT radiodensity (HU) was associated with inferior OS; OS was 5.4 years vs NR in patients with SAT radiodensity in the upper vs middle/lower tertiles, respectively (P=0.001) (Figure 1b) (Table 1). Low muscle radiodensity, reflecting intramuscular fat, was associated with higher ISS and R-ISS stages, older age (>65 and >70 years), anemia (Hb<10 g/dL), and renal failure (Cr>2 mg/dL). High SAT radiodensity, reflecting edema and/or inflammation, was associated with higher ISS and R-ISS stages, anemia (Hb<10), platelets <150 x 109/L, hypercalcemia (Ca>11 mg/dL), renal failure (Cr>2 mg/dL), and high LDH (>222 IU/L); there was no association between skeletal muscle and VAT radiodensity and cytogenetic profile. On multivariate analysis including age >65 years, advanced R-ISS stage (III vs I/II), anemia, thrombocytopenia, hypercalcemia, renal failure, and high LDH, high SAT radiodensity (HU) was independently associated with inferior OS (OS hazard ratio: 1.7, 95%CI; 1.1-2.6, P=0.03), while the association between low muscle radiodensity and OS was no longer statistically significant (OS hazard ratio: 1.5, 95%CI; 1.0-2.3, P=0.07).
Conclusion: Sarcopenia was not associated with OS in patients with newly diagnosed MM. Patients in the upper or lower tertiles for SAT area had decreased OS compared to those in the middle tertile. High SAT radiodensity and low muscle radiodensity were associated with advanced disease stage and adverse laboratory characteristics. High SAT radiodensity was independently associated with decreased OS.
Disclosures
Dispenzieri:Janssen: Membership on an entity's Board of Directors or advisory committees; Oncopeptides, and Sorrento: Other: Data monitoring safety committee; Alynlam, Pfizer, Takeda, and BMS: Research Funding. Dingli:Alexion Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Bristol Meyers Squibb Global Pharmaceutical Company: Consultancy; Novartis Pharmaceuticals: Consultancy; Roche: Consultancy; Sanofi: Consultancy; Regeneron Pharmaceuticals: Research Funding. Lacy:Celgene: Research Funding. Gertz:Sorrento: Other: personal fees; Research to Practice: Other: personal fees; Celgene: Other: personal fees; Johnson & Johnson: Other: personal fees; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: personal fees from Data Safety Monitoring Board; Physicians Education Resource: Other: personal fees; Juno: Other: personal fees; Ashfield: Other: personal fees; Aptitude Healthgrants: Other: personal fees; Janssen: Other: personal fees; Sanofi: Other: personal fees; Prothena: Other: personal fees; Ionis/Akcea: Other: personal fees; i3Health: Other: Development of educational materials for i3Health. Kapoor:Sanofi: Honoraria, Research Funding; X4 Pharmaceuticals: Honoraria; Regeneron: Research Funding; Amgen: Research Funding; Ichnos: Research Funding; Loxo: Research Funding; Karyopharma: Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Research Funding; Casma: Honoraria; Pharmacyclics: Honoraria; Imedex: Honoraria; GSK: Honoraria; Cellectar: Honoraria; Oncopeptides: Honoraria. Kourelis:Novartis: Research Funding. Lin:Bluebird Bio: Consultancy, Research Funding; Takeda: Research Funding; Merck: Research Funding; Gamida Cell: Consultancy; Sorrento: Consultancy; Celgene: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Legend: Consultancy; Vineti: Consultancy; Novartis: Consultancy; Juno: Consultancy. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal